Introduction
Cardiovascular diseases are the leading cause of death worldwide, and India is no exception. As the burden of heart conditions continues to grow, innovative solutions like wearable defibrillators are gaining significant traction. These life-saving medical devices monitor patients at risk of sudden cardiac arrest and deliver a therapeutic shock when necessary.
If you’re a medical device manufacturer in India looking to enter the wearable technology market, understanding the manufacturing process and regulatory requirements is critical. This blog will guide you through what wearable defibrillators are, how they are manufactured, and what standards must be met for market entry in India and abroad.
Looking For a Medical Device Consultant?
Let’s have a word about your next project
What is a Wearable Defibrillators?
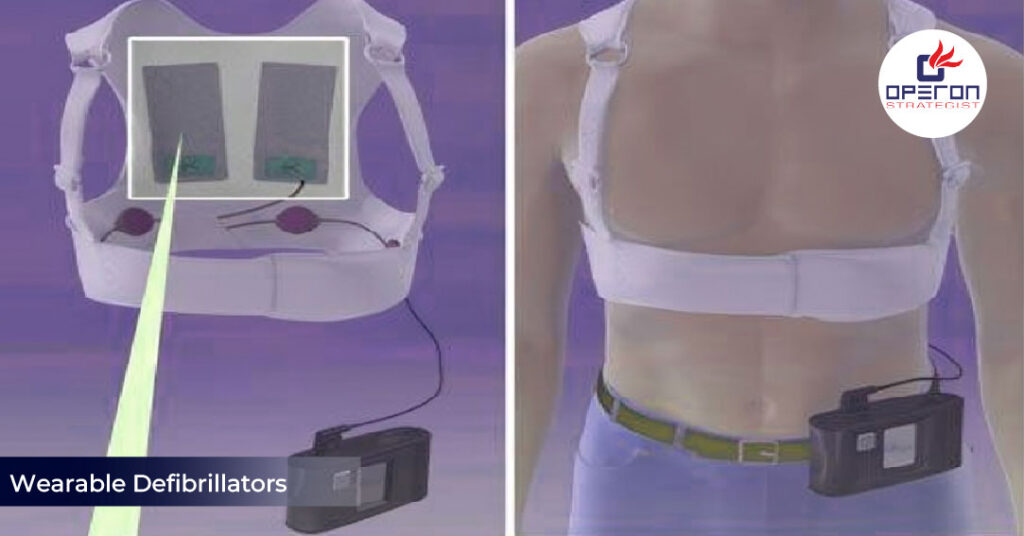
A wearable defibrillator is a non-invasive medical device worn externally by patients at risk of sudden cardiac arrest. It continuously monitors heart rhythms and automatically delivers a shock to restore a normal rhythm when a life-threatening arrhythmia is detected.
These devices are often prescribed to patients who are not candidates for implantable cardioverter defibrillators (ICDs) or are awaiting surgery. Unlike ICDs, wearable defibrillators do not require surgical implantation, making them ideal for temporary use.
Key Components of a Wearable Defibrillator
To manufacture a wearable defibrillator, you must understand its critical components:
- Electrodes/Sensors: Monitor cardiac rhythms and detect arrhythmias.
- Microprocessor & Software: Analyze data and trigger shock when needed.
- Defibrillation Circuit: Delivers electrical shock to restore heart rhythm.
- Battery Pack: Ensures portability and uninterrupted functionality.
- Wearable Vest or Harness: Ensures proper contact with the skin.
- Communication Module: Allows remote monitoring by healthcare providers.
Each of these components must be designed with patient safety, comfort, and compliance in mind.
Manufacturing Process Overview
1. Design & Prototyping
- Conduct user needs analysis based on clinical input.
- Ensure compliance with IEC 60601-1 for electrical safety.
- Develop embedded software per IEC 62304 standards.
2. Component Sourcing
- Source biocompatible and EMC-compliant components.
- Select suppliers with ISO 13485-certified quality systems.
3. Assembly
- Follow Good Manufacturing Practices (GMP).
- Use cleanroom environments where applicable for critical components.
4. Testing & Validation
- Conduct functional, electrical safety, and EMC testing.
- Perform software verification and risk assessments.
- Undertake clinical evaluation or simulated use testing if required.
5. Packaging & Sterilization (If Applicable)
- Use protective packaging to maintain device integrity.
- Ensure compliance with labeling and handling guidelines.
6. Post-Market Surveillance
- Implement complaint-handling procedures.
- Plan for ongoing safety monitoring and regulatory reporting.
Regulatory Requirements in India
In India, wearable defibrillators are regulated by the Central Drugs Standard Control Organization (CDSCO). Manufacturers must comply with the Medical Devices Rules, 2017, and follow a well-defined regulatory pathway to ensure legal manufacturing and marketing.
1. CDSCO Medical Device Classification
Wearable defibrillators are categorized as Class C or Class D medical devices based on their risk profile. Manufacturers need to:
- Apply for a manufacturing or import license via the CDSCO online portal.
- Submit a Device Master File (DMF) and Plant Master File (PMF).
- Ensure compliance with all provisions under the Medical Devices Rules, 2017.
2. Quality Management System – ISO 13485
Implementing an ISO 13485:2016 certified Quality Management System is mandatory for obtaining CDSCO approval and exporting to international markets. This ensures consistent product quality, traceability, and process control.
3. Clinical Evaluation Requirements
Depending on the novelty of the product and available clinical data, manufacturers may be required to conduct a clinical investigation in India or submit existing clinical evidence from global studies.
4. Labeling & Import Norms
All wearable defibrillators must carry CDSCO-compliant labeling, which includes:
- UDI (Unique Device Identification), if applicable
- Manufacturing/import license number
- Storage conditions, batch number, and expiration date
International Regulatory Pathways
United States (FDA)
- Classified as Class III.
- Requires Premarket Approval (PMA) for clinical data submission.
- Must comply with 21 CFR Part 820 (Quality System Regulation).
European Union (EU MDR)
- Regulated under EU Medical Device Regulation (EU MDR 2017/745).
- Requires CE Marking through a Notified Body.
- Clinical Evaluation Report (CER), Post-Market Surveillance (PMS), and Technical Documentation are mandatory.
Key Considerations for Successful Compliance and Manufacturing
Developing a wearable defibrillator is a highly rewarding endeavor, but it requires strategic planning and a structured approach to meet quality and regulatory expectations. Here are important factors to focus on for success:
- Regulatory Preparedness: Understanding device classification and global regulatory frameworks (like CDSCO, FDA, and EU MDR) early in the design process helps streamline approvals and avoid costly redesigns.
- Strong Quality Management System (QMS): Implementing ISO 13485:2016 ensures consistent product quality and positions your organization for both domestic and international success.
- Human-Centered Design: Combining clinical safety with ergonomic design ensures your wearable device is effective, comfortable, and user-friendly — enhancing patient outcomes and satisfaction.
- Cybersecurity & Data Protection: With real-time monitoring features, ensuring data privacy and secure wireless communication builds trust with healthcare providers and patients.
- Scalable Manufacturing Infrastructure: Investing in scalable and compliant manufacturing setups, including clean room environments where needed, prepares your business for long-term growth and export opportunities.
Need Regulatory Support for Your Wearable Defibrillator Project?
How Operon Strategist Can Help
At Operon Strategist, we specialize in helping Indian medical device manufacturers develop compliant, market-ready products. Whether you’re looking to get CDSCO approval, implement ISO 13485, or pursue FDA/CE certifications for wearable defibrillators, we provide:
- Turnkey & regulatory support
- End-to-end QMS implementation
- Technical file and dossier preparation
- Clinical evaluation support
- Design and manufacturing consulting
Contact Operon Strategist today to ensure your product meets all manufacturing and regulatory requirements.