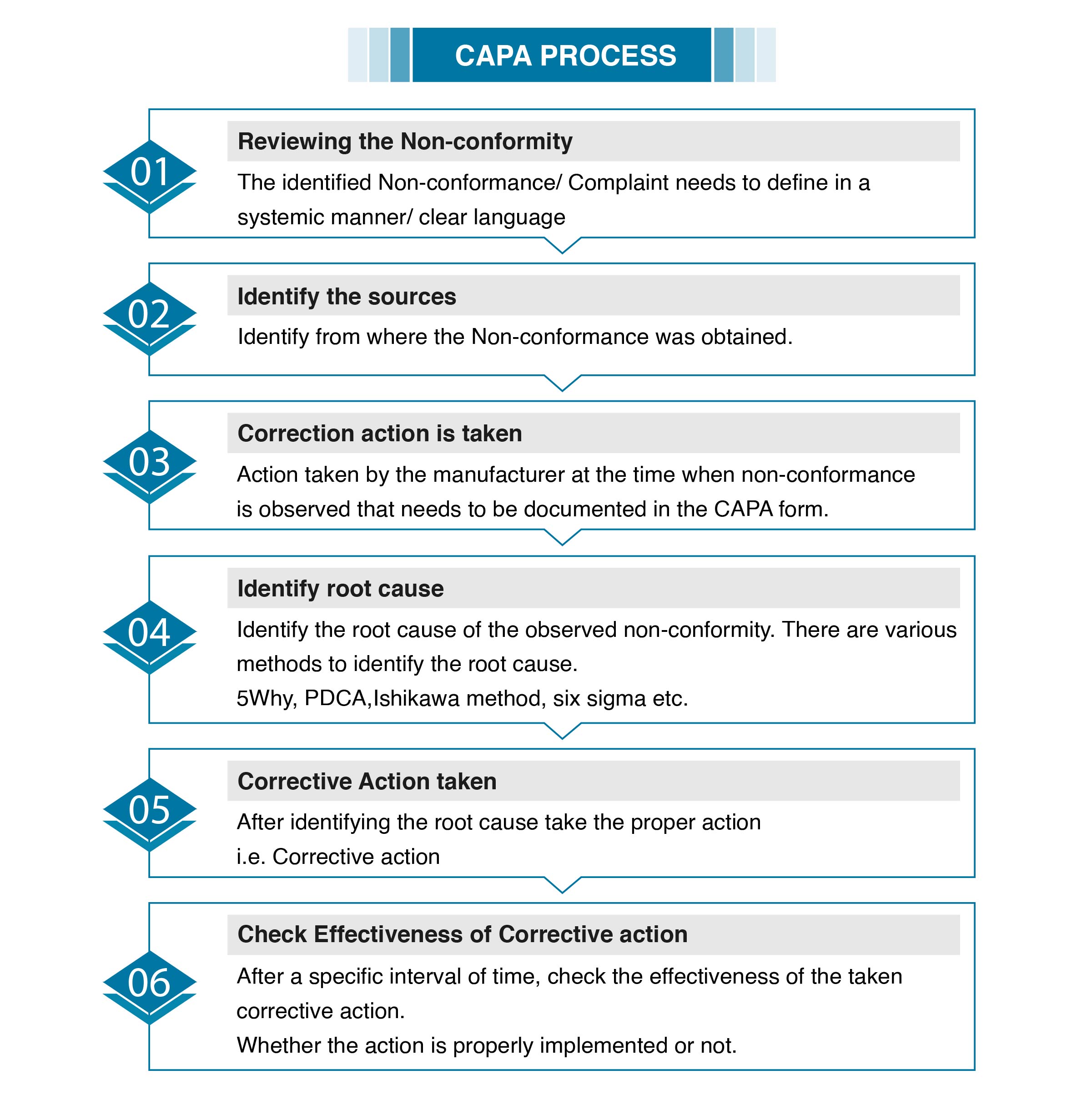
CAPA is acronym of Corrective Action and Preventive Action. The CAPA process is one of the center cycles that exist inside the quality arrangement of every medical device organization. The FDA mandates that medical device organizations break down quality review reports, work activities, returned items, administration records, concessions, processes and work on the root cause of non-conforming products. We team of Operon strategist help our clients to manage CAPA documentation; CAPA creates a huge amount of paperwork , information from processes, work tasks, quality review reports, client grumblings and administration records, meeting notes, CAPA structures, main driver examination archives, and then some. Our team keep documents organized ,open for endorsement and survey as you explore potential CAPA occasions.
- Operon Strategist is ISO 13485 Medical Device Consultant helps to create the documents for ISO 13485 certification. for more details and services contact us or whatsapp us
Likewise with every quality cycle, laying out and reporting arrangements and systems early and frequently is the most ideal way to remain coordinated and successfully carry out the CAPA interaction at your association
What Should Trigger a CAPA?
CAPA is nothing but Corrective and preventive action.
Definitions: –
- Correction: Action to eliminate a detected non-conformity.
- Corrective action: Action to eliminate the cause of nonconformity and to prevent a recurrence.
- Preventive action: Action to eliminate the cause of potential non-conformities in order to prevent their occurrence.
The action taken by the manufacturer in the case of but not limited to Product-related Nonconformity, Process related Nonconformity or other Nonconformities observe in the Audits or Complaints received etc. Taking a CAPA is challenging for a medical device manufacturer. Because non-effective CAPA list of reasons for 483 observation/ warning letters from the FDA, Non-conformities in the audits and many companies struggle to identify when they should even initiate a CAPA.
Purpose of the CAPA?
CAPA process is nothing but the investigation, addressing the systemic quality issues, such as a component that repeatedly fails inspections during manufacturing. Keep this in mind as we get deeper into the different events that may trigger a CAPA. Before going to the actual process check which activities requires a CAPA.
Non-conformance: – Non-conformance (or nonconformity/nonconformance) means that something went wrong with a process, service, or product, and the result does not match the initial specifications.
There are various types of Non-conformances, some are product-related, some are processes related and some non-conformity observed during the audit. However, it’s important to note that not all nonconformances will trigger a CAPA. A non-conformance initiates a process that includes regulating the nonconforming product and researching the cause of the issue as well as the likelihood that it will occur again, similar to a CAPA.
Complaints: - As per FDA Definition complaint is any communication that alleges deficiencies in your product after it has been placed on the market.
As with Non-conformance the complaints also require the investigation process which identifies the issue or cause of complaint. Similar to a nonconformance, a single complaint will probably not trigger a CAPA. Again, you are looking for indications of a systemic issue, as multiple complaints about the same issue with your product. An adverse event or complaints involving injury to a patient may also trigger a CAPA because of its severity.
Audit Non-conformities :- Both internal and external audits may uncover issues that need to be addressed. However, if these issues exist, your goal should be to discover them during internal audits so that you can address them before ISO/ CE audit and FDA Inspection.
The Non-conformities and Observations observe during the audit (both internal and external) require to take immediate action to overcome the observed non-conformance. Before taking a CAPA, you must determine whether the issues discovered during internal audits are systemic. To be thorough, medical device companies would frequently escalate every complaint to CAPA while conducting an internal audit. However, in most cases, it isn’t necessary. While a CAPA may come from any area of your QMS that presents a systemic quality issue, there are a few main areas that often trigger a CAPA:
CAPA- Risk base approach
There should be a strong correlation between the CAPA and risk management in your organization. Before taking the CAPA of the problem you should check whether the same problem is already captured in your risk management document or not, If not then it\’s time to update the documents.
It’s also important to remember that starting a CAPA is a risk management activity. Risk is formed by combining the likelihood of injury and the severity of the harm. As we have seen, CAPAs are typically initiated when a problem is systemic, implying that it has a higher probability of occurring. Nonetheless, due to the seriousness of adverse events involving a patient’s injury, a CAPA may be required.
CAPA is significant for medical device organization for global administrative consistence, cost reserve funds, proficiency, and marking, among different reasons. operon strategist medical device consultancy provides end to end solution and regulatory guidance for medical device manufacturer.
-
adminhttps://operonstrategist.com/author/admin-2/
-
adminhttps://operonstrategist.com/author/admin-2/
-
adminhttps://operonstrategist.com/author/admin-2/
-
adminhttps://operonstrategist.com/author/admin-2/