Design and Development Documentation
Understanding Medical Device Design and Development
Effective medical device design and development are critical for meeting quality expectations and preventing customer complaints and market failures. Poor design and development are often the root causes of customer complaints, recalls, and device failures. Any product failure represents a significant non-compliance issue and can lead to adverse events for users.

The design and development stages of medical devices are crucial for their success. A poorly designed or inadequately defined device may fail to meet regulatory requirements and gain market approval. Even if a product complies with regulations, it may still fail to deliver the intended functionality and benefits expected by the market, resulting in lower market acceptance compared to well-defined products. Medical device design and development encompass more than just conceptualization, solution development, prototyping, and mass production. It requires significant effort to deliver the right healthcare solution that satisfies customer needs.
What is meant by Medical Device Design Control?
Design controls, which are commanded by the FDA, address a formalized way to deal with the advancement of Class II and Class III medical devices. This process incorporates many layers of required documentation that show the FDA precisely how you have accommodated the well-being and viability of your new device. So medical device design and development companies need to classify their products so that the required design control can be done.
Looking for a Medical Device Regulatory Consultant?
Guide to the Medical Device Design and Development Process
During the design and development stage of the medical device process, we provide assistance to various medical device manufacturing industries in Iran to ensure regulatory compliance. After analyzing a new medical device, the next step in its development is the design phase. This stage is crucial, as a flawed design can lead to inefficiency or risk. At this stage, a design control process system is essential.
Design controls are straightforward and rational measures to ensure that the product you develop aligns with your intended specifications and meets the expectations and requirements of your customers.
- Medical device development involves analyzing market needs to define product specifications. Compliance with regulations, such as those set by the FDA, is crucial. Medical devices are classified based on risk and must adhere to both local and global regulations. Standards ensure quality and usability, allowing stakeholders to evaluate equipment effectively.
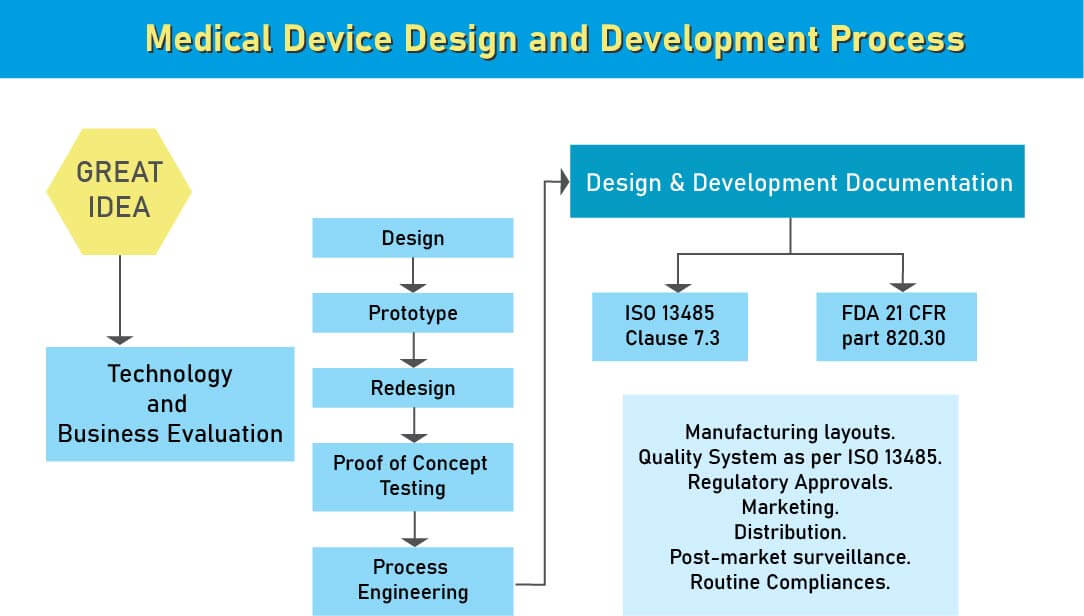
- The International Organization for Standardization (ISO) also provides guidelines for medical device standards. ISO 13485 is a widely recognized standard worldwide for quality management in medical devices. In addition to these global standards, there are region-specific standards, each of which is derived from international standards with slight modifications and adaptations.
- Medical device manufacturers must adhere to Design Control regulations to ensure compliance with regulatory bodies such as the FDA, European Commission, and others, guaranteeing the safety of potential users before devices are marketed. The Design Control process begins with the development and approval of Design Inputs, which outline device design and manufacturing procedures to be implemented during production. Design control is a comprehensive approach that extends beyond the transition of design to the production stage once finalized. It also influences manufacturing processes in response to design stage modifications or post-production feedback. This ongoing process aims to develop a product that is user-friendly and considers iterative changes from user feedback and analysis of failed products for product enhancement.
Why is Medical Device Design Important?
When it comes to medical device development, the absence of comprehensive design and development documentation covering all the stages of the design of a product, is not just a setback, it can be a permanent barrier to getting to market.
For those serious about developing a medical device that can make it through to launch, giving early consideration to the overall governance of the design elements of your project and how it will be recorded is essential. This includes creating a functioning system of design control before you begin that will guide, manage and document the progress of your project from ideation to the start of development and beyond.
What is Product Development in Medical Devices?
The process of medical product development encompasses the entire journey of creating a medical device, starting from identifying a market opportunity to ultimately selling the final tested and certified product. This process involves multiple stages, including opportunity identification, market analysis, concept generation and selection, product design, device engineering, commercialization, and post-market surveillance.
Medical Device Design Services & Product Development Process?
Designing and developing a medical device involves the following key steps:
1. Identify a need and conduct research.
2. Generate concepts and assess feasibility.
3. Define specifications and requirements.
4. Develop detailed designs and prototypes.
5. Test and validate the device.
6. Ensure regulatory compliance.
7. Establish manufacturing processes.
8. Prepare for commercialization.
9. Monitor post-market performance and feedback.
By following these steps and integrating design control and quality management principles, you can successfully create a medical device that meets user needs and regulatory standards.
Our Role in Device Design Control:
Operon Strategist plays a pivotal role in the design and development of documentation for medical devices, providing expert guidance and support throughout the process. They offer regulatory assistance, conducting thorough reviews to ensure compliance with FDA regulations or CE marking requirements. Operon also assists in template development for standardized documentation, offers compliance guidance, and provides training to ensure teams understand the importance of proper documentation practices. Overall, the Operon Strategist ensures that design and development documentation is accurate, comprehensive, and aligned with regulatory standards, facilitating the successful development and commercialization of safe and effective medical devices. Contact us for further details.
