FDA 21 CFR Part 820 Quality System Regulation
FDA 21 CFR Part 820 delineates the FDA’s regulations governing Current Good Manufacturing Practice (CGMP) for quality systems. These regulations form the foundation of Quality Management Systems (QMS), ensuring the production of safe, effective, and compliant medical products. Compliance with 21 CFR Part 820 is imperative for selling medical devices in the US market, and our team is here to assist you throughout this process. We offer QMS solutions tailored to help you align with the latest regulations and standards, ensuring smooth navigation of the compliance journey.
What is FDA 21 CFR Part 820 Quality system Regulation?
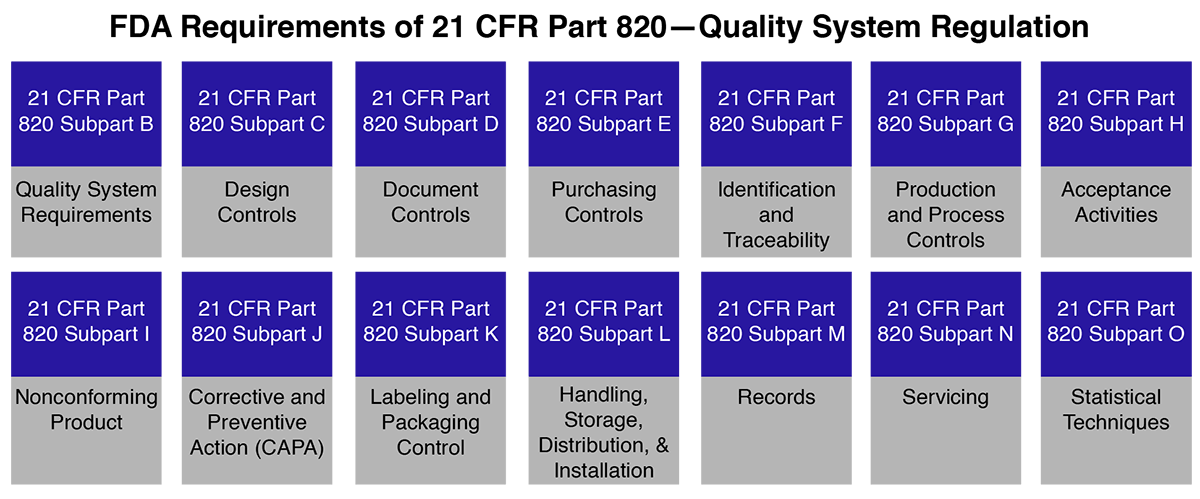
Let’s first understand CFR. CFR is the code of Federal, in which 21 is the title, 800 represents the series, which is for medical devices, and 820 is for quality system regulation (QSR), on which we are spreading light.
FDA 21 CFR Part 820 medical device covers the processes used in and the facilities and controls used for the design, manufacture, packaging, labeling, storage, installation, and servicing of medical devices. Manufacturers are inspected by the US FDA as per Part 820; however, there is no certification process for Part 820, and only compliance with the requirements is assessed.

FDA QSR Compliance for Medical Device Manufacturers:
As we know 21 CFR part 820 is part of CGMP I.e Current Good Manufacturing Practices regulations. The quality systems for FDA-regulated products (food, drugs, biologics, and devices) are known as Current Good Manufacturing Practices. CGMP requirements for devices in part US FDA 21 CFR Part 820 (21 CFR part 820) were first authorized by section 520(f) of the Federal Food, Drug, and Cosmetic Act, FDA 21 CFR part 820 (QSR 21 CFR part 820 is USFDA current good manufacturing (CGMP) requirements for medical device manufacturers.
The FDA 21 CFR part 820 also known as Quality System Regulation i.e., FDA QSR outlines current good manufacturing practice (CGMP) regulations that control the techniques used in and the efficiency and controls used for, the manufacturing, labeling, packaging, storage, installation, design, and servicing of all finished medical devices predetermined for human use and marketing in the USA. The above requirements are turned to ensure that medical devices are safe and effectively produced by medical device manufacturers who support FDA (Food and Drugs Administration) inspections to assure FDA QSR (quality system regulation) 21 CFR part 820 compliance.
Why Should You Care About 21 CFR Part 820?
Medical device manufacturers should take care of the 21 CFR Part 820 regulation because if they are found to fall short of the minimum standard in the inspection process, they might receive a warning letter from the FDA. This can cause huge reputational damage and negatively impact your market performance. When you are importing devices from the US to Iran to place in the market, it is advisable to check whether they are fulfilling the QSR requirement or not.
Operon Strategist Role in FDA as a 21 CFR Part 820 Consultant
Are you ready to enter the US market with your medical devices? If so, compliance with the US FDA’s Quality System Regulation (QSR), specifically known as 21 CFR Part 820, is essential. Even if you already have a quality management system in place, adherence to this regulation is mandatory before selling your device. Similarly, if you’re expanding into the Iran market while manufacturing devices in Iran, you’ll need to meet the requirements set by the Iran market. Our team is equipped to guide you through the compliance process.
Operon Strategist begins by conducting an initial gap analysis of your existing system to assess the level of development of your quality system. We offer 21 CFR 820 training courses, during which we assist clients in documentation and effective implementation across various functions of the company. Additionally, we conduct mock audits to evaluate the implementation of Part 820 requirements. Post-inspection, we guide to help clients address any observed non-conformities. FDA 21 CFR Part 820 empowers manufacturers to establish and adhere to quality systems, ensuring consistent compliance with applicable requirements and specifications for their products.
Get Expert Consulting Services for FDA 21 CFR Part 820 (Quality System Regulations)
Why Choose Operon Strategist?
Operon Strategist is a medical device regulatory consultant and helps the clients to register SBU (Small Business Unit), if applicable. Take out the testing requirement of the product, creation of the dossier, resolving the queries and after completion of all the activities, the client receives the US FDA 510 k approval.
We are a medical device consulting firm assisting companies and medical device manufacturers by providing consultancy services that support the registration of drug-device combination products. We have experience with each constituent part and the GMP regulations that together form the basis for their development and manufacture: Drug (21 CFR 210 and 211), Device (FDA 21 CFR part 820) and Combination Products (21 CFR Part 4). Our experience and work methodology differentiated us from others. To know more details and to avail ourselves of our services you easily contact us.
