Clean Room Design Consultant for Medical Devices
Regulatory Aspects of Clean Room Design
As a Clean Room Design consultant, we provide expert clean room design solutions to medical device manufacturers. A clean room is a facility ordinarily utilized as a part of specialized industrial production or scientific research.

What is a Clean Room?
Cleanrooms are the enclosed area within manufacturing facility, these are specially designed rooms to control air pollutant level, humidity and personal access to meet environmental conditions. Clean Room helps in establishing & maintaining an environment with a low level of environmental pollutants such as dust, airborne microbes, aerosol particles & chemical vapors. For that, clean room design is under the expertise is more important.
Typically, Manufacturers/producers of medical devices, pharmaceuticals, and biotechnology products need clean rooms for their units.
Why Do Medical Device Manufacturers Need Clean Rooms?
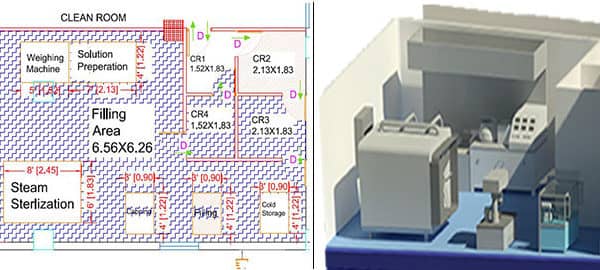
Clean Room Design enables the clients to have a perspective on the total project extension and complete cost before the project starts.
The point of the clean room design contract worker must give a total facility and at least support by the client or end client.
When appropriately designed and constructed, clean rooms are exceptionally effective execution machines.
What are the medical device clean-room regulations?
ISO 13485 is an international standard that ensures the patients’ safety by regulating the cleanliness control of medical products. ISO is a non-governmental body that works in 164 countries and enforces standards for a variety of commercial markets. ISO has set different standards for different purposes. As an ISO 13485 medical device consultant, We know how to implement the proper QMS system and guide our clients in clean room design.
How Does Operon Strategist Help You in Clean Room Designing Consultations?
In our role as Clean Room Design Consultants, we specialize in providing comprehensive services encompassing clean room validation and design. Our solutions adhere strictly to international standards and norms, ensuring optimal performance and compliance. Whether you’re establishing a new manufacturing unit or seeking regulatory support for your facility, our expertise is at your disposal. With a track record of successful clean-room project completions, we offer AutoCAD clean-room design services tailored to various industries including manufacturing, scientific labs, R&D, and primary packaging.
What Is Operon Strategist’s Role in Clean Room Designing?
We as Clean Room Design Consultants, guide Medical Device manufacturers on supporting elements to maintain their clean room conditions suitable for manufacturing like entry-exit procedures, gowning procedures, etc. Operon Strategist helps in the structure of the Medical Device clean room according to the ideal degrees of air quality. Moreover, controlling the air molecule check, environmental factors, for example, humidity, temperature, and weight are additionally considered while structuring a clean room. Our group of cleanroom design consultants will prompt assessment and proposals for short-range, medium-term, and long-haul plan goals and upgrades.
Our Clean Room Consulting Services Include:
- Document survey of plans and details adjusting mistakes during clean room design and building before its real development
- Building appointing administrations to guarantee that the office is working as it was planned in the clean room configuration stage and introductory prerequisites.
As clean room design consultants, we need to ensure the various sizes and unpredictability that are required for structuring and to keep up low degrees of air particles according to the clean room standard ISO 14644-1. The clean room design consultants design the whole air circulation framework, including arrangements for sufficient downstream air returns. In vertical stream rooms, this implies the utilization of low-divider air returns around the edge of the zone.
Our clean room design consultants are focused on furnishing you with the most accurate and up-to-date regulatory expertise in the business. We are cleanroom design consultants who help you in the design, layout, and conceptualization phases of your next cleanroom establishment project! We have already guided many manufacturers or medical device service providers in Iran.
In Brief, Operon Strategist is a medical device regulatory consulting company that provides regulatory advisory & guidance to various manufacturers in the healthcare industry to ensure the strategic development of these manufacturers. We are one of the leading regulatory consultants working closely with various regulatory authorities like SFDA, EDA, and MHRA. To implement a proper QMS system for your manufacturing unit or design a clean room you can freely contact us. We also provide Medical Devices and Clean Room Design consultation for India, the USA, Saudi Arabia & Egypt.
