Inside Medical Device Manufacturing: The Materials That Matter
Ever wondered what makes medical devices safe, effective, and long-lasting? In medical device manufacturing, the choice of materials is everything. Whether it’s an implant, a surgical tool, or a diagnostic device, each material is carefully selected for its biocompatibility, durability, and cost-effectiveness. These materials ensure that medical devices perform flawlessly where it matters most—saving lives and improving healthcare.
Inside 11 Key Medical Devices: The Materials Shaping Modern Healthcare
Looking For a Medical Device Regulatory Consultant?
Let’s dive into the most commonly used materials, why they’re essential, and how they shape the future of medical innovation. Let’s explore what’s behind the devices that keep us healthy!
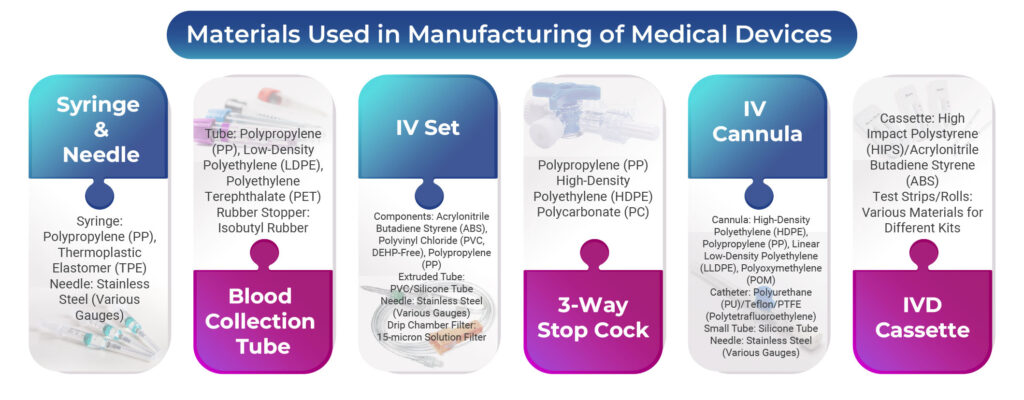
1. Materials Used In Syringe & Needle Manufacturing:
- Syringe: Polypropylene (PP), Thermoplastic Elastomer (TPE)
- Needle: Stainless Steel (Various Gauges)
Polypropylene (PP) is a popular plastic due to its durability and resistance to chemicals. It is often used in syringe barrels and plungers. Thermoplastic Elastomer (TPE) provides a flexible and comfortable grip, enhancing user experience. Stainless Steel needles, available in various gauges, offer strength and precision for injections.
Also, read Disposable Syringe Manufacturing Process and Machineries
2. Materials Used In Blood Collection Tube (BCT) Manufacturing:
- Tube: Polypropylene (PP), Low-Density Polyethylene (LDPE), Polyethylene Terephthalate (PET)
- Rubber Stopper: Isobutyl Rubber
Polypropylene (PP), LDPE, and PET are used for the tubes due to their clear visibility, chemical resistance, and durability. Isobutyl Rubber stoppers provide an airtight seal, preventing contamination and preserving sample integrity.
Chemicals:
- Clot Activator with Gel
- K2/K3 EDTA
- Sodium Citrate
- Sodium Fluoride
- Potassium Oxalate
- Lithium Heparin
- Sodium Heparin
These chemicals stabilize blood samples for various tests, ensuring accurate and reliable results.
Read about Blood Collection Tubes Manufacturing
3. Materials Used In IV Set Manufacturing:
- Components: Acrylonitrile Butadiene Styrene (ABS), Polyvinyl Chloride (PVC, DEHP-Free), Polypropylene (PP)
- Extruded Tube: PVC/Silicone Tube
- Needle: Stainless Steel (Various Gauges)
- Drip Chamber Filter: 15-micron Solution Filter
ABS and DEHP-Free PVC ensure safety and compatibility with various IV solutions. Silicone Tubes offer flexibility, while stainless steel needles ensure safe and effective delivery of fluids.
Related article – IV Set Manufacturing
4. Materials Used In 3-Way Stop Cock Manufacturing:
- Polypropylene (PP)
- High-Density Polyethylene (HDPE)
- Polycarbonate (PC)
These materials are chosen for their durability and ability to withstand the pressures of fluid management in medical settings.
Know About 3-Way Stop Cock Manufacturing
5. Materials Used In IV Cannula Manufacturing:
- Cannula: High-Density Polyethylene (HDPE), Polypropylene (PP), Linear Low-Density Polyethylene (LLDPE), Polyoxymethylene (POM)
- Catheter: Polyurethane (PU)/Teflon/PTFE (Polytetrafluoroethylene)
- Small Tube: Silicone Tube
- Needle: Stainless Steel (Various Gauges)
The combination of these materials provides flexibility, strength, and biocompatibility for effective intravenous access.
6. Materials Used In IVD Cassette Manufacturing:
- Cassette: High Impact Polystyrene (HIPS)/Acrylonitrile Butadiene Styrene (ABS)
- Test Strips/Rolls: Various Materials for Different Kits
HIPS and ABS offer robustness and clarity for diagnostic devices.
7. Materials Used In Blood Bag Manufacturing:
- Bag: Polyvinyl Chloride (PVC), High-Density Polyethylene (HDPE), Polypropylene (PP)
- Needle: Stainless Steel
- Rubber: Synthetic Rubber
These materials ensure safe storage and transfusion of blood.
Blood Bag Manufacturing – Regulatory Aspects
8. Materials Used In Gauze Bandage Manufacturing:
- Cotton Fabric
Cotton is chosen for its absorbency and softness, ideal for wound care.
Surgical Bandage Manufacturing
9. Materials Used In Gloves Manufacturing:
- Latex/Vinyl/Nitrile
Each material provides different levels of flexibility, comfort, and protection for medical procedures.
Read About How is Medical Glove Manufacturing Done?
10. Materials Used In Surgical Gown Manufacturing:
- Non-Woven Fabric
Non-woven fabric is lightweight, breathable, and provides an effective barrier against contaminants.
Know Everything About Disposable Medical Gowns
11. Materials Used In Urine Bag Manufacturing:
- Polyvinyl Chloride (PVC), Polypropylene (PP)
PVC and PP provide durability and flexibility for fluid collection.
Understanding the materials used in medical devices helps ensure the right choices are made for safety, efficacy, and comfort in healthcare applications.
For All-in-one Medical Device Regulatory Consulting Services
Ensuring Quality & Innovation in Medical Device Materials
The materials used in medical devices aren’t just components—they’re the foundation of safety, durability, and effective treatment. As technology advances, smarter material choices will continue to enhance healthcare. But selecting the right materials is only part of the equation—meeting global regulations is just as critical. That’s where Operon Strategist comes in.
We help manufacturers navigate complex regulatory landscapes, from CE marking and FDA 510(k) clearance to ISO 13485 compliance and cleanroom validation. With our expertise, you gain a trusted partner for seamless approvals, quality assurance, and market success. Let’s shape the future of healthcare together!

- Operon Strategisthttps://operonstrategist.com/author/snehal/
- Operon Strategisthttps://operonstrategist.com/author/snehal/
- Operon Strategisthttps://operonstrategist.com/author/snehal/
- Operon Strategisthttps://operonstrategist.com/author/snehal/