Medical Device development
Medical device development is the process of designing, creating, and bringing to market a device intended for medical purposes. It involves activities such as concept generation, design, prototyping, testing, regulatory approval, and manufacturing.
Medical device development seems to be a complex process with rigid requirements for FDA approval. Medical device professionals with significant expertise in the field of devices can comprehend and divulge rule pertaining to such goods to qualify in the market medical device development should address below 5 phases of development, while developing the devices QMS plays vital role, and as 21 CFR part 820 QSR consultant we are guiding them to implement effective QSM for their organization.
Looking for Medical Device Consultation?
What are the Stages Phases in Medical device development?
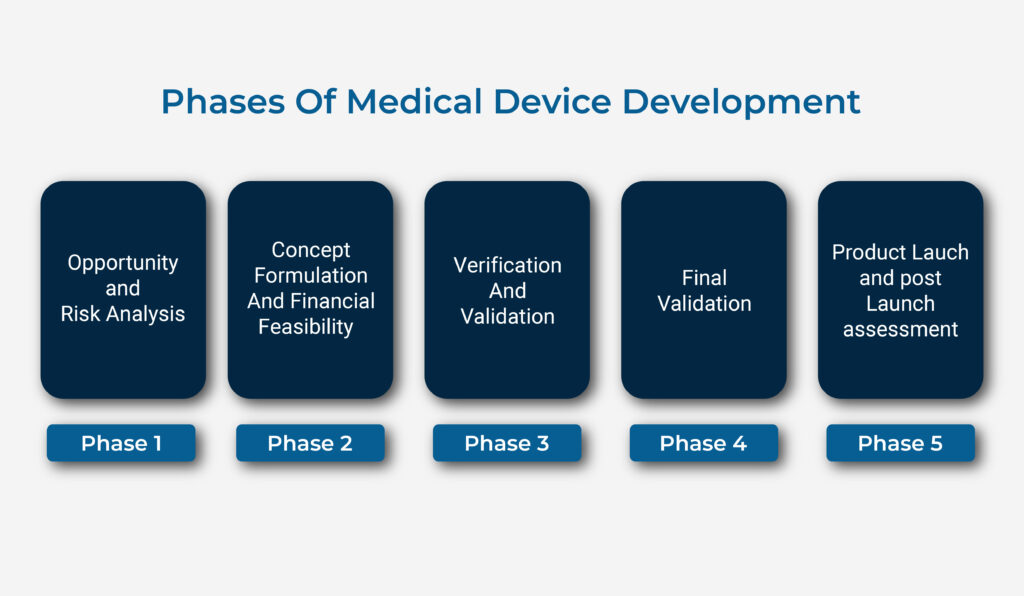
The different legislative requirement and standards may impact the phases of medical device development. we are summarizing five phases of medical device design here :
- Phase I: Opportunity and Risk analysis:
for any medical device development risk analysis can be the first step to encash the opportunities. Risk analysis helps us to determine whether we proceed to next phase or not? this, phase works on design and development plan, risk benefit analysis, risk management plan etc.
- Phase II : Concept formulation and feasibility:
Once you are persuaded that your device has a market position, is practical and monetarily achievable, at exactly that point set the ball moving. You will require subsidizing for prototyping and trialing your device. This phase illustrates feasibility of your idea in various ways. Feasibility focuses on engineering design work, determining the key material and components, reviewing certifications etc. as far as product concept goes your plan should work on the incomplete work, the regulatory requirements, which will clear your path .
- Phase III : Verification And Validation
Now your device is beginning to take shape, you have a prototype, you have done some trials but you haven’t really put the design through its paces, you are about to go into validation and verification of your device and prove that it will really withstand all the pressures of the real world. You must perform a series of validation to ensure user need and intended use of the device are met
- Phase IV: Final Validation
Once you complete the above steps you are now came to a point where you are gathering all evidences of testing such as biocompatibility, electrical safety etc whichever applicable. After completing overall study, the technical documentation gets ready for submission. To create a technical file team of operon strategist can guide you and can submit your file to correct notified body . The NBs will review your technical file and also audit DHR, DMR and DHF. For any assistance in DHR, DMF you can freely contact us.
- Phase V: Product Lauch and post Launch assessment
Once the notified body certify your product and your QMS you can market your product.one need to verify their plan according to the regulatory requirement. Once you place your product on market you need to gather users feedback data regularly as part of risk management process .as per EUMDR these activities termed as PMCF and PMS ( Post market surveillance ).
This procedure of ideating, planning and building up a medical device is enormously perplexing, with many moving parts, documentation prerequisites and regulatory obstacles to survive. We have recorded just a couple of the procedures and necessities vital to the five phases, however they all show how focal great archive the board will be to your inevitable achievement. A well managed QMS will help you to easily meet the regulatory demands. These 5 phases of medical device development make the whole profitable for you and for the other stalk holders ..
FAQs
[rank_math_rich_snippet id=”s-24eebbb1-7f81-43f1-ada1-b707b2a8e96f”]




