CDSCO Manufacturing License for Medical Devices
What is a CDSCO Manufacturing License for Medical Devices?
The Central Drugs Standard Control Organization (CDSCO) is India’s national regulatory authority responsible for regulating the approval, manufacturing, import, sale, and distribution of medical devices. Under the Medical Devices Rules, 2017 (MDR 2017), any company intending to manufacture medical devices in India must obtain a CDSCO Manufacturing License.
This license ensures that the manufacturer complies with all quality, safety, and regulatory requirements mandated by the Indian regulatory framework.
Regulatory Basis of the Manufacturing License
The CDSCO manufacturing license is issued under the CLAA Scheme (Competent Authority Licensing Agreement). The authority responsible for granting the license is the State Licensing Authority (SLA), which evaluates compliance with MDR 2017, including facility readiness, documentation, QMS requirements, and product classification.
Looking for CDSCO Manufacturing License?
How to Register for CDSCO Manufacturing License?
To register for a CDSCO manufacturing license in India, determine the specific license type, ensure your facility meets eligibility criteria and GMP guidelines, employ qualified technical staff, and submit a comprehensive application, including Form 27, prescribed fees, site master file, premises details, and manufacturing process information to the relevant State Drug Control Department or CDSCO, depending on the type of license required.
Get expert guidance throughout this process from Operon Strategist, a medical device manufacturing license consultant who can provide valuable assistance and streamline your application procedure for your medical device manufacturing license.
If you are seeking guidance and support with regulatory compliance in the Indian medical device industry, look no further! As a CDSCO consultant, we can provide expert advice and assistance with all aspects of regulatory affairs.
Operon Strategist is a medical device regulatory consultant with over 12 years of experience and completed over 200 CDSCO registrations. For more details, please reach us @ WhatsApp or call 919403892834
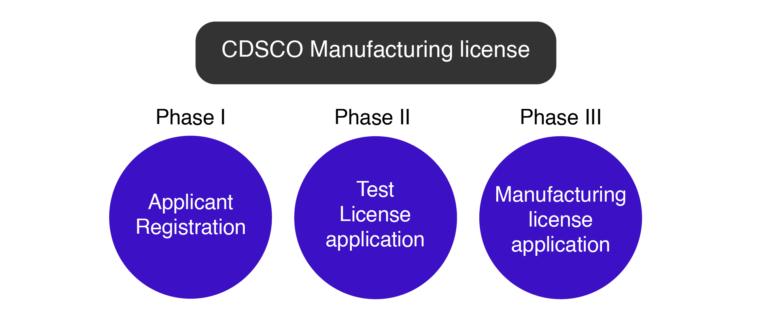
What are the Phases of CDSCO Manufacturing License?
Phase I – Applicant Registration for CDSCO Manufacturing License
In the first phase of manufacturing license CDSCO registration, login credentials are to be generated on the CDSCO portal by uploading the required documents.
Document requirements for CDSCO login credentials:
Documents required for this stage include address proof like the certificate of registration or certificate of incorporation or Import-export certificate or MTNL/BSNL bills of the corporate site, and ID proof of an authorized person, this person can be any person apart from the management team who will be authorized to all types of registration.
Procedure:
- CDSCO Online form submission.
- Uploading documents and company details.
Once the form is submitted, preliminary approval mail will be received from CDSCO then another mail stating hard copy submission will be received.
Phase II – CDSCO Manufacturing Test License Application
CDSCO manufacturing test license is required to manufacture small quality medical devices for testing, evaluation, demonstration, and training of personnel.
Procedure:
- Apply via an online portal
- Form MD-12 filled with correct details
- Uploading documents.
- Fees payment.
- Change in status of the application.
Phase III – CDSCO Manufacturing License Application.
The CDSCO manufacturing license application is required to manufacture medical devices for commercial purpose
Procedure:
- Online application submission on the CDSCO portal
- For manufacturing Class A & Class B devices MD-3. and for Class C and D devices MD-7 Forms should be filled with the correct details.
- Uploading documents.
- Fee payments as per CDSCO
- Receiving mail about approval or rejection of license.
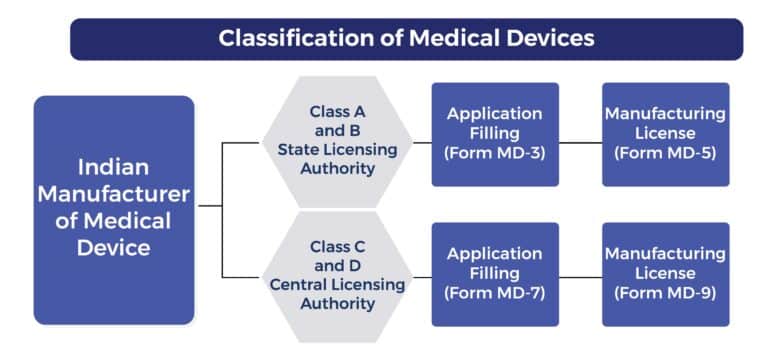
What is the Classification of Medical Devices in CDSCO?
The Central Drugs Standard Control Organization (CDSCO) in India classifies medical devices into four categories based on the level of risk they pose to patients and users. The classification system is based on the intended use of the device and the potential harm it could cause if it malfunctions.
Classification of medical devices as per Indian CDSCO:
- Class A: – Low-risk medical devices such as stethoscopes, bandages, and other basic medical instruments that have minimal or no potential to harm patients or users.
- Class B: – Low-to-moderate-risk medical devices such as blood pressure monitors, syringes, and needles that may cause harm to patients or users if they malfunction, but the harm is not life-threatening.
- Class C: – Moderate-to-high-risk medical devices such as artificial heart valves, orthopedic implants, and catheters that have the potential to cause serious harm or injury to patients if they malfunction.
- Class D: – High-risk medical devices such as pacemakers, heart-lung machines, and ventilators that are critical to the health and survival of patients and could cause serious harm or death if they malfunction.
Step-by-Step Procedure to Obtain CDSCO Manufacturing License for Medical Device
To get CDSCO manufacturing license for a medical device in India, here is a step-by-step procedure to follow:
- Determine Your Medical Device’s Classification: The CDSCO categorizes medical devices into four classes based on their risk level. Your application’s classification will determine the level of scrutiny required.
- Prepare Technical Documentation: Create technical documentation for your medical device, which should include the device description, manufacturing process, device specifications, safety, and performance data. Check that the documentation meets the CDSCO requirements.
- Submit the Application: Submit the CDSCO’s application for a manufacturing license. The technical documentation, a cover letter, and the required fees must all be included in the application.
- CDSCO review: The CDSCO will examine your application and technical documentation. If the CDSCO requires additional information, it may issue a deficiency letter.
- Inspection of Manufacturing Facility: After reviewing your application, the CDSCO will inspect your manufacturing facility. The inspection will determine whether your facility complies with CDSCO regulations.
- Manufacturing License: The CDSCO will grant you a manufacturing license if your manufacturing facility meets the CDSCO requirements.
- License Renewal: A manufacturing license is typically valid for five years. You must renew your license before it expires.
Overview of CDSCO Forms MD 3, MD 5, MD 7, and MD 9 for Medical Device Registration
CDSCO Form MD 3 and MD 5
The Central Drugs Standard Control Organization (CDSCO) regulates the import, manufacture, sale, and distribution of medical devices in India. Two crucial forms in this regulatory framework are Form MD 3 and Form MD 5. These forms are essential for obtaining necessary licenses to operate legally within the medical device industry in India.
Form MD 3 is the application form used to obtain a license to manufacture Class A or Class B medical devices for sale or distribution. Class A and Class B devices are typically low to moderate-risk devices.
Form MD 5 is the license issued to a manufacturer for the production of Class A or Class B medical devices. Once an application (typically using Form MD 3) is reviewed and approved by the CDSCO, the manufacturing license is granted through Form MD 5. This license certifies that the manufacturer is authorized to produce specified medical devices in accordance with the Medical Devices Rules, 2017.
CDSCO Form 7 and MD 9
CDSCO Form MD 7 and MD 9 pertain to the regulation of medical devices in India under the Medical Devices Rules, 2017. They are part of the regulatory framework that governs the importation and distribution of medical devices.
Form MD 7 is the application form for obtaining a license to manufacture Class C or Class D medical devices for sale or distribution.
Form MD 9 is the license to manufacture for sale or distribution of Class C or Class D medical devices.
Expert Assistance for Getting CDSCO Certificate for Medical Devices
How to Apply on CDSCO Portal for Manufacturing License of Medical Device?
To apply for a manufacturing license for a medical device in India on the CDSCO (Central Drugs Standard Control Organization) portal, you will need to follow the below steps:
- Create an account on the CDSCO online portal using your email address.
- After registering, log in to the portal and go to the “Online Application” section.
- Select “Medical Devices” in the “Online Application” section, and then the type of license you want to apply for, i.e., manufacturing license.
- Fill out the application form with the necessary information, such as the details of the CDSCO md, manufacturing facility, quality control procedures, and other pertinent information.
- Upload the CDSCO-required documents, such as the application form, manufacturing plan, and product specifications.
- Use the portal’s online payment gateway to pay the application fee.
- Submit your application form and wait for the CDSCO to review it.
- The CDSCO will issue the manufacturing license for your medical device once your application is approved.
It is essential to ensure that you meet all the necessary requirements and have all the required documents before submitting your application to the CDSCO.
Simplify Your CDSCO Manufacturing License
With 12+ years of expertise and 200+ successful registrations, we handle everything—classification, documentation, and approvals. Let us save you time and streamline the process for MD 5, and MD 7 licenses.
Ready to start? Fill out the form and let’s make it hassle-free!
Mail Us:
Call Us Now:
Looking For a Medical Device Regulatory Consultant?
FAQs
How do I get medical device manufacturing license in India?
You can get manufacturing license in India by CDSCO (Central Drugs Standard Control Organisation) The applicant must apply for Manufacturing License to sell or distribute medical devices. The applicants must use the online portal of the Ministry of Health of India
Who issues the CDSCO manufacturing license?
The license is issued by the State Licensing Authority under the supervision of CDSCO.
How long is the CDSCO manufacturing license valid?
The manufacturing license is valid for 5 years, subject to compliance.
Do I need a manufacturing license for assembling or packaging imported components?
Yes. Final assembly, packaging, or sterilization in India requires a CDSCO manufacturing license.