Manufacturing Plant Layout Design Consultant for Medical Devices
Efficient Manufacturing Plant Layout Design for Medical Devices
The first step in building a successful manufacturing facility is creating a thoughtful and efficient plant layout. For medical device manufacturers, this step is crucial as it forms the backbone of regulatory compliance and operational excellence. Every medical device manufacturing facility must adhere to strict guidelines issued by authorities like the US FDA, CDSCO, SFDA, and CE Marking to meet industry standards for safety and quality.
If you manufacture orthopedic implants, disposable devices, primary packaging materials, or pharmaceutical products, your manufacturing unit must comply with GMP (Good Manufacturing Practice) requirements and additional regulatory standards.
The Blueprint for Success:
- Streamlined Material & Workflow Design: Prevent mix-ups and ensure operational efficiency.
- Dedicated Zones for Product Segregation: Maintain quality by reducing risks of cross-contamination.
- Cleanroom-Ready Classified Areas: Fulfill critical cleanroom requirements for advanced medical device production.
- Logical Process Flow Design: Align steps to ensure smooth transitions and eliminate bottlenecks.
Looking for Manufacturing Plant Layout Design Consultant?
Fill the Form or Mail Us to: enquiry@operonstrategist.com
Why is Manufacturing Plant Layout Design Critical for Medical Devices?
In the highly regulated world of medical devices, precision and compliance are non-negotiable. A professionally designed manufacturing plant layout serves as the foundation for operational excellence. It not only enhances efficiency and productivity but also guarantees alignment with strict regulatory requirements and quality standards.
By adopting an optimized layout, businesses can streamline workflows, minimize compliance risks, and ensure readiness for audits, all while setting a robust framework for long-term success in the competitive medical device industry.
Advantages of Professional Manufacturing Plant Layout Design Services for Medical Devices
- Seamless Compliance: Effortlessly meet global standards, including US FDA, CE Marking, and GMP regulations.
- Optimized Efficiency: Streamline operations to minimize downtime and maximize output.
- Boosted Productivity: Achieve faster production cycles while maintaining top-notch quality.
- Exceptional Quality Control: Implement robust systems to ensure consistent product excellence.
- Uncompromised Safety: Design layouts that prioritize the well-being of staff and product integrity.
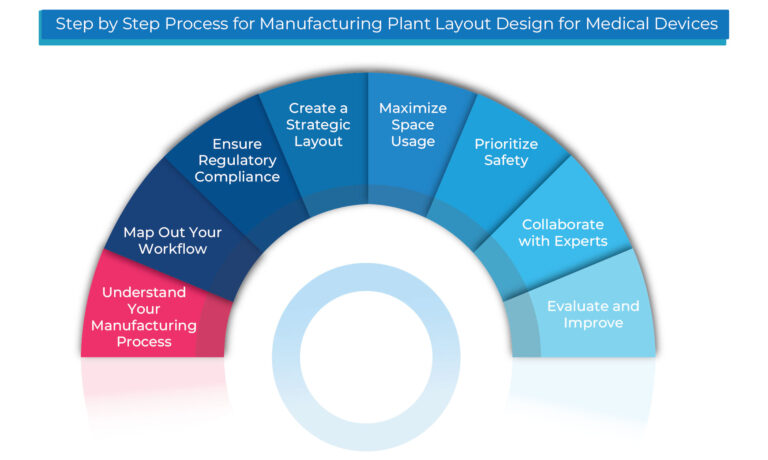
Step By Step Process for Manufacturing Plant Layout Design for Medical Devices:
Designing the perfect manufacturing plant layout for medical devices involves careful planning, ensuring regulatory compliance, cleanliness, and efficient workflows. Here’s an easy-to-follow process to guide you:
- Understand Your Manufacturing Process: Get familiar with every stage of production.
- Map Out Your Workflow: Plan a smooth, efficient process from start to finish.
- Ensure Regulatory Compliance: Adhere to all necessary standards, like US FDA, CE Marking, and GMP.
- Create a Strategic Layout: Design your plant layout for optimal performance.
- Maximize Space Usage: Use available space efficiently to boost productivity.
- Prioritize Safety: Implement safety measures for your team and products.
- Collaborate with Experts: Work with engineers and architects for precision.
- Evaluate and Improve: Regularly assess and improve the layout to stay compliant and efficient.
Following this process will help you build a compliant and efficient facility designed for medical device manufacturing success.
Transform Your Medical Device Manufacturing Facility With Operon Strategist Expert Consultation!
Why Choose Operon Strategist for Your Medical Device Manufacturing Layout?
- Specialized Expertise: Operon Strategist specializes in creating optimized layouts for medical device manufacturing facilities.
- Regulatory Compliance: We ensure your plant layout meets all regulatory standards, while boosting efficiency and productivity.
- Bespoke Design Solutions: Our team crafts personalized layouts, tailored specifically to your unique needs and challenges.
- Comprehensive Review: We conduct a thorough evaluation of your current layout, pinpointing key areas for enhancement.
- Seamless Implementation: From concept to completion, we provide end-to-end support for your manufacturing setup.
- Enhanced Operational Performance: Our designs optimize workflow, ensure safety, and drive overall plant efficiency.

Get in touch with us today to kickstart your medical device manufacturing project and leverage our team’s expert insights!
Simplify Your Manufacturing Facility Process
With 12+ years of expertise and 200+ successful certifications, we handle everything—compliance, technical documentation, and MHRA approvals. Let us save your time and streamline the UKCA marking process for your medical devices.
Ready to start? Fill out the form and let’s make it hassle-free!
Mail Us:
Call Us Now:
Let's Connect! Your Queries, Our Expertise!
FAQs
What are the major steps involved in the manufacturing of a medical device?
Step 1: Device Discovery and Concept. Step 2: Preclinical Research-Prototype. Step 3: Pathway to Approval. Step 4: FDA Device Review. Step 5: FDA Post-Market Device Safety Monitoring.
What are the 3 procedure for layout of plants?
Determine space requirements and allocate activity areas. Develop plot plan and block plan i.e. integrate all plant operations. Develop detailed layouts and plan building along with its arrangement. Evaluate, modify and check the layouts
How do you design a manufacturing plant layout?
1.Stakeholder interviews. 2.Surveying and/or architect consultation. 3.Process and packaging design. 4.Staff and component movement analysis. 5.Review of other associated space requirements. 7.Line layout development. 8.Line computer simulation and budget preparation.